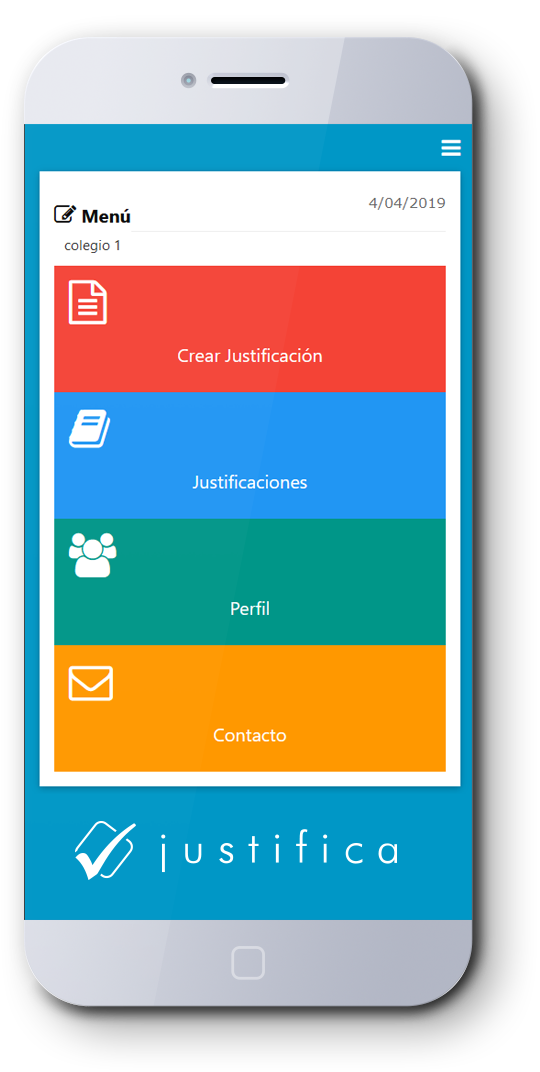
Actualmente, el sistema es lento, inseguro y engorroso, ya que tienes que enviar por agenda la comunicación e incluso el certificado médico, el cual muchas veces se pierde. Otro gran problema radica en la falta de comunicación con el profesor, ya que éste muchas veces no se entera del verdadero motivo de la inasistencia del alumno hasta que recibe la libreta con la justifican en la libreta, incluso muchos días después.
Con Justifica podrás hacerlo de manera rápida, segura y fácil, con solo un par de clicks y desde tu teléfono móvil, lo que facilitara el proceso para ti y para el colegio.
Todos los derechos reservados JUSTIFICA Santiago-Chile 2024